Liquefaction of Hydrogen Gas. Cyrogenic Temperature Production
Introduction
Liquefaction of gases is an important part of cryogenics and the gases that are used as cryogens include Nitrogen, Hydrogen, Helium, Oxygen and so forth. The basic fundamental principle behind liquefying these gases is since they have a very low boiling point; once they are liquefied they can be used to generate very low temperatures if allowed to boil by absorbing heat from the surroundings. In this article we will study about the liquefaction process of Hydrogen gas.
Joule Thomson Effect
Before delving further into studying about the liquefaction of hydrogen, I want to explain a phenomenon known as the Joule’s Thomson effect which is closely associated with the liquefaction of gases.
You must know that as per thermodynamics a perfect gas will not have any change in temperature when it is subjected to irreversible expansion, but on a more philosophical note nothing is perfect in this universe and this applies to gases as well. Hence real gases do experience a decrease in temperature when subjected to free expansion. This is so because in case of real gases, there are forces between the molecules and they are not perfect point particles but have some volume as well.
But this cooling only happens if the particular gas which is being expanded is below what is known as its inversion temperature, which is nearly -77 degrees Celsius for Hydrogen. So this means that if hydrogen is to be liquefied by throttling it has to be below this temperature.
Liquefaction of Hydrogen
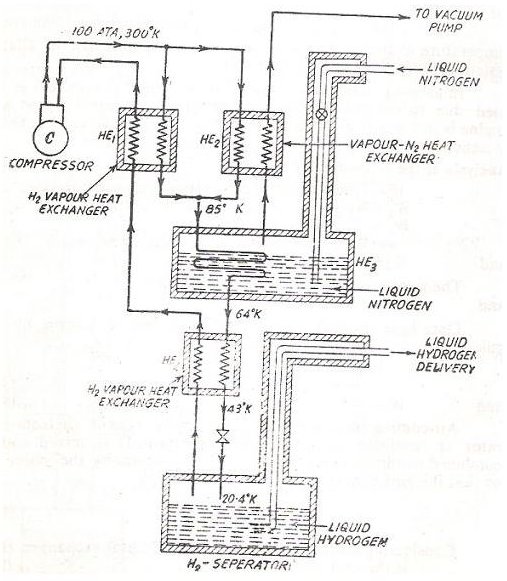
Several techniques can be used for liquefaction of gases and described below is an arrangement which is used to liquefy Hydrogen gas. Take a thorough look at the picture below and then proceed to read on the description as follows.
The typical values of temperature and pressure along various points on the circuit have been given in the circuit itself and are self explanatory so I will just explain the fundamental flow of the process.
The basic principle is based on the Joule’s Thomson effect described above. Firstly pure Hydrogen gas is compressed to a high pressure and obviously its temperature also rises. It is important to use pure Hydrogen as impurities could solidify at the later stages causing problems in the overall system.
The hot pressurized gas is then passed through two heat exchangers and again combined to be passed through a tank containing liquid nitrogen, finally to pass through another heat exchanger.
Now we have gaseous Hydrogen which is it a low temperature but high pressure and it is perfect time to throttle it and receive it in the receiver from where it can be delivered as per usage.
References
Image of Hydrogen Liquifier - Arora, S.C. & Domkundwar, S. (1993_). A Course In Refrigeration & Air-Condioning._ Delhi: Dhanpat Rai & Sons